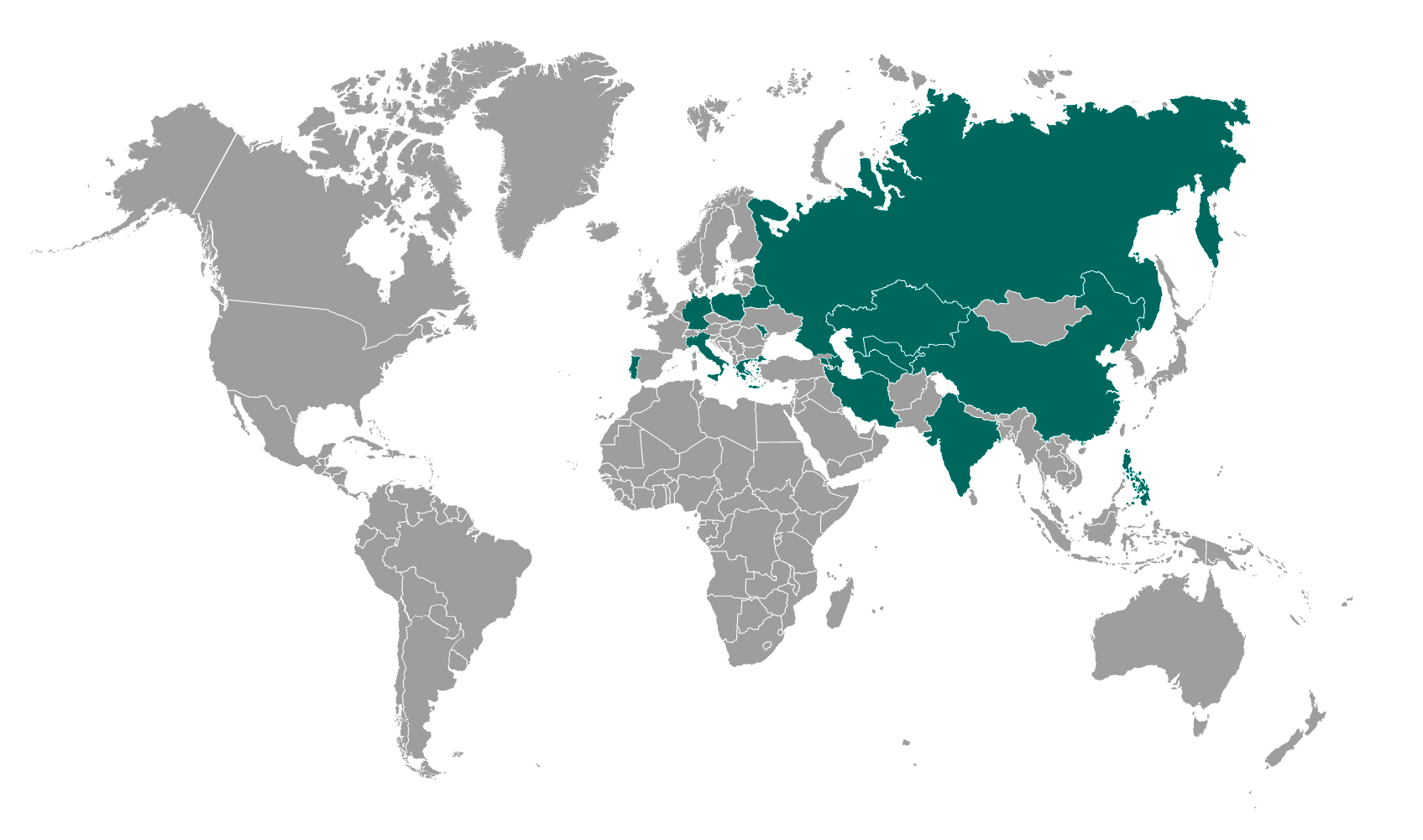
Amolab in the World: the distribution network is growing
Distribution agreements concluded in the Russian Federation, Belarus, Kazakhstan, Armenia, Tajikistan, Uzbekistan and Philippines, after those reached in China and Iran. The process of registration has begun in the United States, while a partnership in Greece is already established.
Through the signing of agreements with several countries that have shown interest in SensUS Lung and SensUS Touch, the international distribution network of Amolab devices has been consolidated.
The Apulian company has started the registration phase in the United States, where it is necessary to obtain FDA registration (Food and Drug Administration): a complex and laborious path, for which the company can rely on the work of a ‘catalyst’, a Dutch entity that embraces two universities and three commercial facilities, to which the Lecce CNR spin-off has been admitted thanks to the EIT (European Institute of Innovation & Technology) Health’s Bridgehead Global programme.
The EIT Health project
The programme is aimed at European companies in the health sector with the objective of supporting them in expanding their business beyond national and European markets through facilitators.
There are three objectives of Eit Health: to strengthen health systems in Europe and worldwide, to promote the improvement of people’s health and to contribute to a sustainable health economy.
Observing the US model, Amolab wants to register both devices, aiming to solve the clinical needs of the exigent American market, both from an obstetrical-gynaecological and pneumological point of view and covid-19 diagnosis.
Amolab broadens its horizons
In July 2021, Amolab signed agreements that will enable the distribution of innovative technologies supporting the medical community in the Russian Federation and five Central Asian countries – Belarus, Kazakhstan, Armenia, Tajikistan, Uzbekistan – as early as the end of 2022.
In this area, it is planned to start distribution for SensUS Touch.
The agreements follow those signed to establish the distribution network in Iran and China. In Iran, the network covers SensUS Touch and is already active because the country recognises the validity of CE certification. In order to enter the huge market of China, Amolab is currently working on obtaining the NMPA registration required by law for medical devices, and then distributing both SensUS Touch and SensUS Lung.
Amolab is also present in Greece: in recent months, in fact, a collaboration network has been set up concerning the use of SensUS Lung in hospital facilities.
The expansion of channels on an international scale is an important recognition of the validity of Made in Italy technology in the field of medical research.